Product Pipeline
Opportunities for Orphan Indications
Multiple Antibody Formats
Multiple Ways to Arm the Antibody
Anti-GPC-1 Antibodies for Therapeutics and Imaging
Our current objective is to target orphan indications that can expedite market access for the company.
See GlyTherix Patent Estate and Peer Reviewed Publications.
Our patented antibody targets Glypican-1 (GPC-1), a protein overexpressed in prostate, pancreatic, bladder, glioblastoma, esophageal and other solid tumors.
We have completed a First-in-H
The trial was a FIH study to evaluate the safety and tumor targeting of Miltuximab® in patients with advanced prostate, bladder and pancreatic cancer. Preclinical studies demonstrated that Miltuximab® accurately targets prostate, pancreatic and bladder cancer cells and is well-tolerated and highly specific in mouse models of prostate cancer.
The trial was conducted at the Macquarie University Hospital and involved manufacturing and clinical management experts from the Australian Nuclear Science and Technology Organisation (ANSTO), AusPep, and Macquarie Medical Imaging.
In July 2018 the Company announced that it has completed enrolment and dosing of all 12 patients in its pioneering clinical trial and was pleased to report Miltuximab® was well tolerated with no
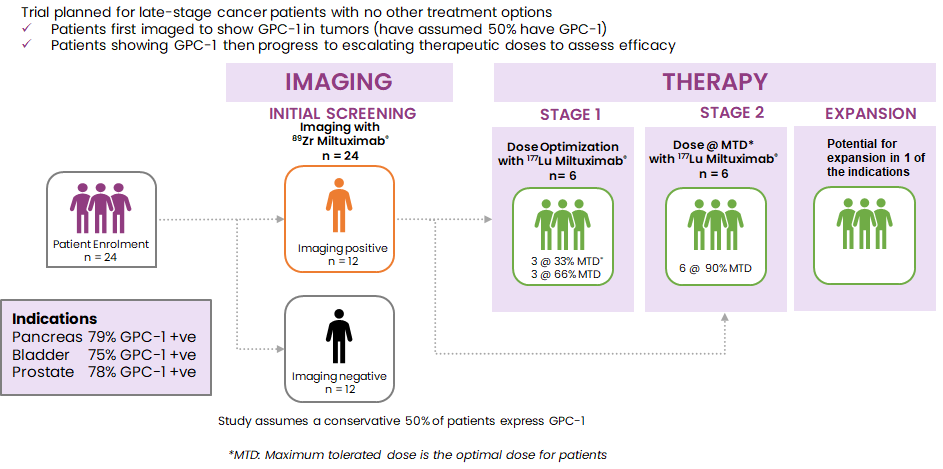
We are currently planning a follow up Phase-1b theranostic study of at least 12 more patients. Candidates will be selected by PET imaging using 89Zr-Miltuximab® and then dosed with the therapeutic version of Miltuximab® (177Lu-Miltuximab®).
Glytuzumab® is a humanized version of Miltuximab® and also targets human GPC-1. The Glytuzumab® program is currently undergoing Lead Optimisation and Lead Selection. Several humanized leads have been generated with similar binding affinities as that of the parent molecule Miltuximab®. The final Glytuzumab® lead selected will be taken forward from cell line development through to large scale GMP production for future clinical trials.
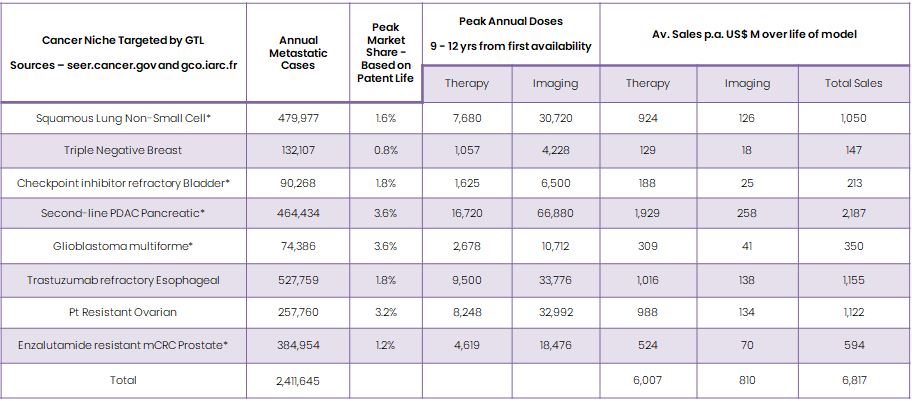
Total Addressable Market (Global) – Lead Indications
Phase 1b – Time from First to Last Patient – 10 Months

Global research collaborations
GlyTherix’s collaborations are advancing trials of cancer therapeutics, and imaging as well as building a future product pipeline.
For example:
- The ARC Hub for Advanced Manufacture of Targeted Radiopharmaceuticals, AMTAR
- The Centre for Advanced Imaging at the University of Queensland
- The Australian Prostate Cancer Research Centre at the Queensland University of Technology
Comparison of PSMA and GPC-1 Technologies Useful in Prostate Cancer Diagnosis and Therapy
The success of an antibody therapeutic relies on its specific expression in tumor, with limited or no expression in normal tissue. In prostate cancer, Prostate Specific Membrane Antigen (PSMA) has been targeted clinically for both imaging and therapy. Overexpressed in the prostate tumors of some individuals, it has allowed successful imaging using 68Ga-PSMA-PET and therapy using 177Lu-PSMA or 177Lu-J591. However, not all patients tumors express PSMA, leaving 10-30% of patients with late stage metastatic disease who do not respond to PSMA-directed therapy.
Moreover, PSMA is not only expressed in tumor tissue, but is also expressed in a variety of normal tissues. Immunohistochemistry studies have shown PSMA to be expressed in the kidney, testis, ovary, brain, salivary gland, small intestine, lacrimal glands, colon, liver, spleen, breast, skeletal muscle and benign fractures, as well as malignancies of these tissues (Farag et al, 2020), which means that targeting PSMA using radiation leads to exposure of normal tissue to radiation and subsequent tissue damage and associated side effects.
Glypican-1 is a new tumor targeting molecule that is expressed in a variety of deadly solid tumors of high unmet need (including prostate, brain, bladder, esophageal, pancreas, mesothelioma and cervical). What makes Glypican-1 such a promising therapeutic target is its lack of expression in normal tissue (confirmed by the FIH clinical trial completed with Miltuximab®). Moreover, targeting of Glypican-1 with antibody therapeutics has proven completely safe in numerous animal studies, as well as the human study of Miltuximab®.
Clinically, GPC-1 is a particularly exciting target, as expression of GPC-1 has been associated with poor clinical prognosis and aggressive tumors, in several solid tumors including pancreatic, esophageal and glioblastoma. Furthermore, we have evidence to suggest potential synergy between GPC-1 directed therapy and standard of care therapies such as chemotherapy and radiotherapy.