Anti-GPC-1 Antibody for Therapeutic & Imaging Applications
Our patented antibodies target Glypican-1 (GPC-1), a protein overexpressed in prostate, pancreatic, bladder, glioblastoma, esophageal, lung and ovarian cancers. Importantly, there is no antibody reactivity in normal tissues.
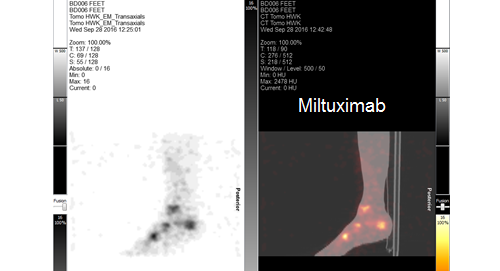
We have now completed a First-in-Human trial in Australia using Miltuximab®, our anti-GPC-1 monoclonal antibody (ANZCTR registry). The trial dosed 12 patients and no drug-related adverse events were observed.
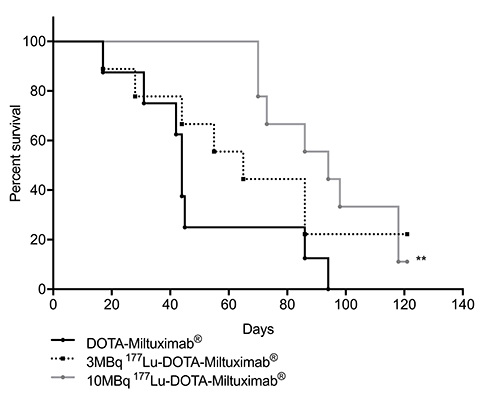
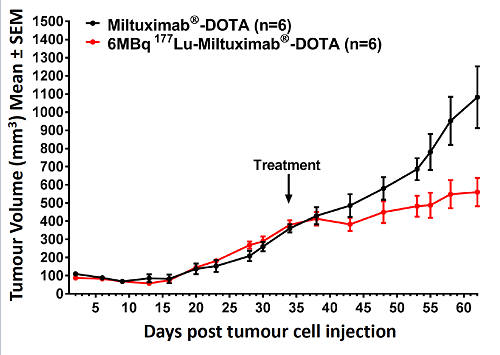
We are currently raising funds to run a Phase-1b theranostic study of at least 12 more patients. Candidates will be selected by PET imaging using 89Zr-Miltuximab® and then dosed with 177Lu-Miltuximab® , the therapeutic version.
Glytuzumab® refers to the humanized version of Miltuximab® and also targets human GPC-1. The Glytuzumab® program is currently undergoing Lead Optimization and Lead Selection. Several humanized leads have been generated with similar binding affinities to that of the parent molecule Miltuximab®. The final Glytuzumab® lead selected will be taken forward from cell line development through to large scale GMP production for future clinical trials.
Achievements
2023
GlyTherix selected to participate in NYU Endless Frontier Labs program
GlyTherix signs Lutetium-177 supply agreement with ANSTO
2022
High-Yield GMP-Compliant Primary Cell Bank Established for Miltuximab®
2021
Commenced production of GMP-Miltuximab® for Phase-1b clinical trial
2020
Carina Biotech and GlyTherix enter into CAR-T partnership
GlyTherix’s Miltuximab® antibody becomes the flagship project for a Co-operative Research Centre Project Grant
2019
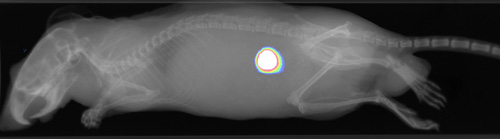
Preclinical work now completed for upcoming Phase I clinical trial in GPC-1 expressing solid tumours. This will use Miltuximab® labelled with either 89Zirconium for imaging or 177Lutetium for therapy
First-in-Human clinical trial of Miltuximab® labelled with 67Gallium for imaging met primary endpoint of safety in all 12 patients
Antibody composition of matter patents granted EU and US