Date, July 21 2022: Sydney.
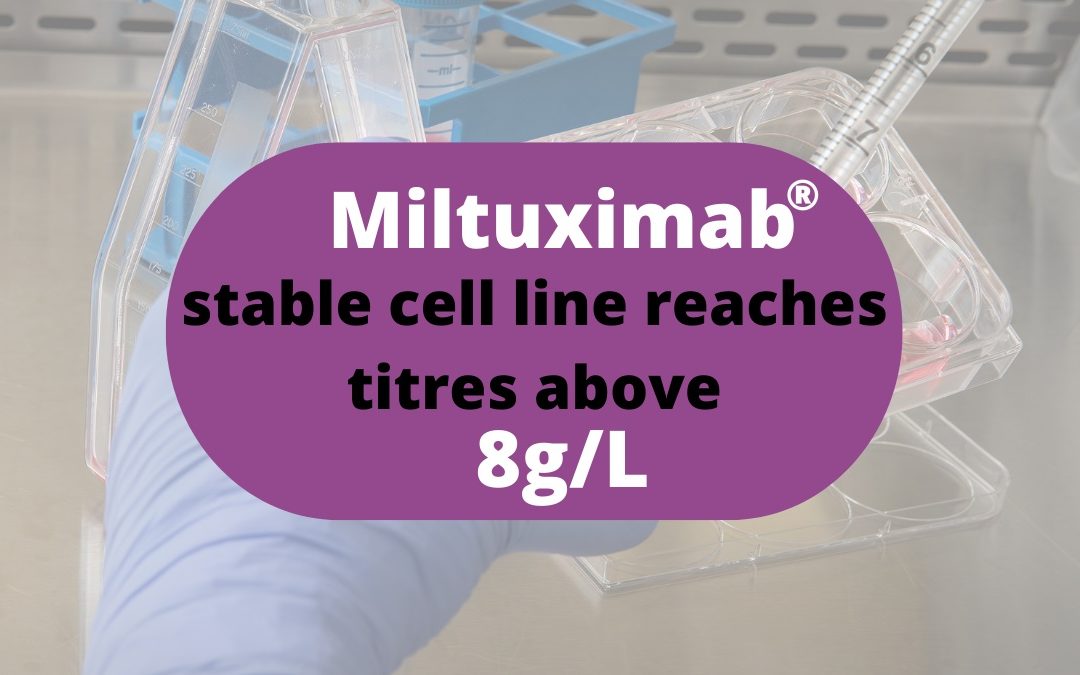
GlyTherix is pleased to announce the successful completion of its high-yield GMP-grade stable cell line development program for its proprietary antibody Miltuximab® in partnership with international biologics manufacturer GenScript ProBio (New Jersey, USA).
Establishing a stable, high-yield cell line suitable for commercial production of clinical-grade monoclonal antibody is a critical milestone for the Company. The Chinese Hamster Ovary (CHO) cell clones selected for Miltuximab® cell banking have reached titres of above 8g/L. Work is now continuing on the process development program for scale-up of Miltuximab® manufacturing at 100L-bioreactor scale for the Company’s upcoming Australian Phase I trial.
Miltuximab® will be used in a Phase Ib trial as an antibody theranostic. Antibody labelled with 89-Zirconium will be used as an imaging agent to select patients showing tumor targeting. This will be followed by patients being offered Miltuximab® labelled with 177-Lutetium as a therapeutic dose.
To find out more about GlyTherix’s technology and clinical program, visit www.glytherix.com
GlyTherix is seeking investors who would like to participate in the company’s Series A funding round.
This project received grant funding from the Australian Government.
Ends
About GlyTherix
GlyTherix Ltd is an Australian immuno-oncology company specializing in developing antibody radiopharmaceuticals for solid tumors. Miltuximab® specifically targets GPC-1, a protein found in solid tumors such as prostate, bladder, pancreatic, glioblastoma, oesophageal and ovarian cancers, and is not present in healthy tissue.
The company has a strong proprietary and Intellectual Property position covering both Miltuximab®, its anti-GPC-1 antibody and the antigen itself, GPC-1. This provides robust and long-term protection for the commercialization of important new treatments to people with little hope. GlyTherix has completed a ‘First-in-Human’ trial of 12 patients using Miltuximab® with no drug-related adverse. GlyTherix is interested in partnerships or collaborations with larger biotech and pharmaceutical partners.
About GenScript ProBio
GenScript ProBio is the subsidiary of GenScript Biotech Corporation, proactively providing end-to-end CDMO service from drug discovery to commercialization with proactive strategies, professional solutions and efficient processes in cell and gene therapy (CGT), vaccine, biologics discovery and antibody protein drug to accelerate drug development for customers. GenScript ProBio has established companies in the United States, the Netherlands, South Korea, Shanghai, Hong Kong, Nanjing and other places to serve global customers, and supported customers in the United States, Europe, Asia Pacific and other regions to obtain more than 30 IND approvals.
Toward the mission of “Innovation through Collaboration”, GenScript ProBio is committed to helping customers shorten the timeline for the development of biological drugs from discovery to commercialization, significantly lowering R&D costs and building a healthier future. Visiting https://www.genscriptprobio.com/ for more information.